Explain the Differences Between the Fda and the Dea
Terms in this set 10 FDA. C ASE 2 As the medication production increased there was a need to develop from CHEM 1212 at Gwinnett Technical College.

The Dea Quota System Food And Drug Law Institute Fdli
What is the major difference between the FDA and DEA.

. Significant changes in wording used in the new law will require companies to rationalize their portfolios and perform a global impact. Drug schedules are categories of drugs regulated by the US. Responsible for approval and removal of product on the market.
Why you need to know the difference. Start studying FDA vs DEA. FDA licenses and inspects legal drugs foods and additives.
A drugs schedule influences how the drug can be legally manufactured imported distributed possessed or used in the United States. DEA was created in an effort to create a single federal agency to enforce the federal drug laws and co-ordinate and consolidate the governments control activities. In determining into which schedule a drug or other substance should be placed or whether a substance should be decontrolled or rescheduled certain.
DEA is an enforcement agency dealing with. The FDA will write you letters. Time needed for executing an input string is less.
Drug Enforcement Agency DEA because any prescription for a controlled substance requires a valid DEA number. Legitimate drug handlers need a DEA Registration. Heres what you need to know.
NFA is easier to construct. They often work together particularly at the headquarters level. Dispensing medications to the public.
The DEA classifies drugs based upon their acceptable medical use and the drugs abuse or dependency potential. For example Schedule I drugs have a high potential for abuse and the potential to. The Drug Enforcement Administration was created on July 1 1973 by President Richard Nixon through an Executive Order consequent to the Reorganization Plan No.
Concerned with general safety standards in the production of drugs food and cosmetics. One of the requirements of the Pharmacy Technician Certification Exam PTCE is the ability to understand and validate the registration numbers issued by the US. Addiction DEA Drug Schedules.
The FDA is a governmental body that licenses and inspects legal drugs additives and supplements. The new regulation is four times longer and contains five more annexes than its predecessor the Medical Device Directive MDD. FDA DEA and EPA.
The manufacturer of a drug. The word safety appears 290 times in the MDRThe MDD by comparison uses it only 40 times. Definition of Controlled Substance Schedules.
Inquiries regarding DEA activities may be sent to the Drug Enforcement Administration Office of Diversion Control 8701 Morrissette Drive Springfield VA 22152. Hospitals and pharmacies are challenged to play a pivotal role in the nations drug supply chain. Government charged with enforcement of more than 200 categories of federal laws.
The DEA is a federal law enforcement agency dealing with drug crimes. A state or local government agency. Education and Welfare herein referred to as FDA do hereby jointly agree to the following terms and conditions as stated herein.
FDA 101FDA regulates foods. Below well explain what DEA numbers are used for how to decode them. The Controlled Substances Act was passed in 1970 to help the United States Drug Enforcement Administration DEA enforce drugs which could pose a risk to society if used improperly.
Answer 1 of 2. NFA rejects the string in the event of all branches dying or refusing the string. The FBI is a primary law enforcement agency for the US.
Those drugs with high risk and no counterbalancing benefit are banned from medical practice and are Schedule I drugs. Drug Classifications Schedule I II III IV V. Did Obama Just Admit That Marijuana Should Be Legalized.
Drug Schedules Drugs substances and certain chemicals used to make drugs are classified into five 5 distinct categories or schedules depending upon the drugs acceptable medical use and the drugs abuse or dependency potential. A public interest group concerned with drug abuse. To identify the authorities and roles for cooperative efforts between the two agencies subject to this memorandum in establishing uniform Federal standards.
Conversely those considered to have the lowest risk would be in Schedule V 5. Were going to see a lot more consumer tech devices get the FDAs blessing. The ABCs of Pharmacy Compliance.
Under the CSA a controlled substance is any drug with a potential for abusedrugs which do not pose an addiction risk are not regulated by the CSA although they are by other. Learn vocabulary terms and more with flashcards games and other study tools. DFA rejects the string in case it terminates in a state that is different from the accepting state.
An updated and complete list of the schedules is published annually in Title 21 Code of Federal Regulations CFR 130811 through 130815. DEA Drug Schedules. The DEA is enforcement regulatory and scientific.
The DEAs drug schedule organizes drugs into groups based on risk of abuse or harm. The abuse rate is a determinate factor in the scheduling of the drug. Drugs and other substances that are considered controlled substances under the Controlled Substances Act CSA are divided into five schedules.
Between The Food and Drug Administration. Specific enforcement activities include actions to. Enforcement FDA vs DEA.
DEA will put you in prison. The Drug Enforcement Administration or DEA classifies drugs based on several factors. The objective of FDA regulatory programs is to assure compliance with the Federal Food Drug and Cosmetic Act the Act.
Time needed for executing an input string is more. A medical society or association. The FDA is strictly regulatory and scientific.
FDA approved vs.
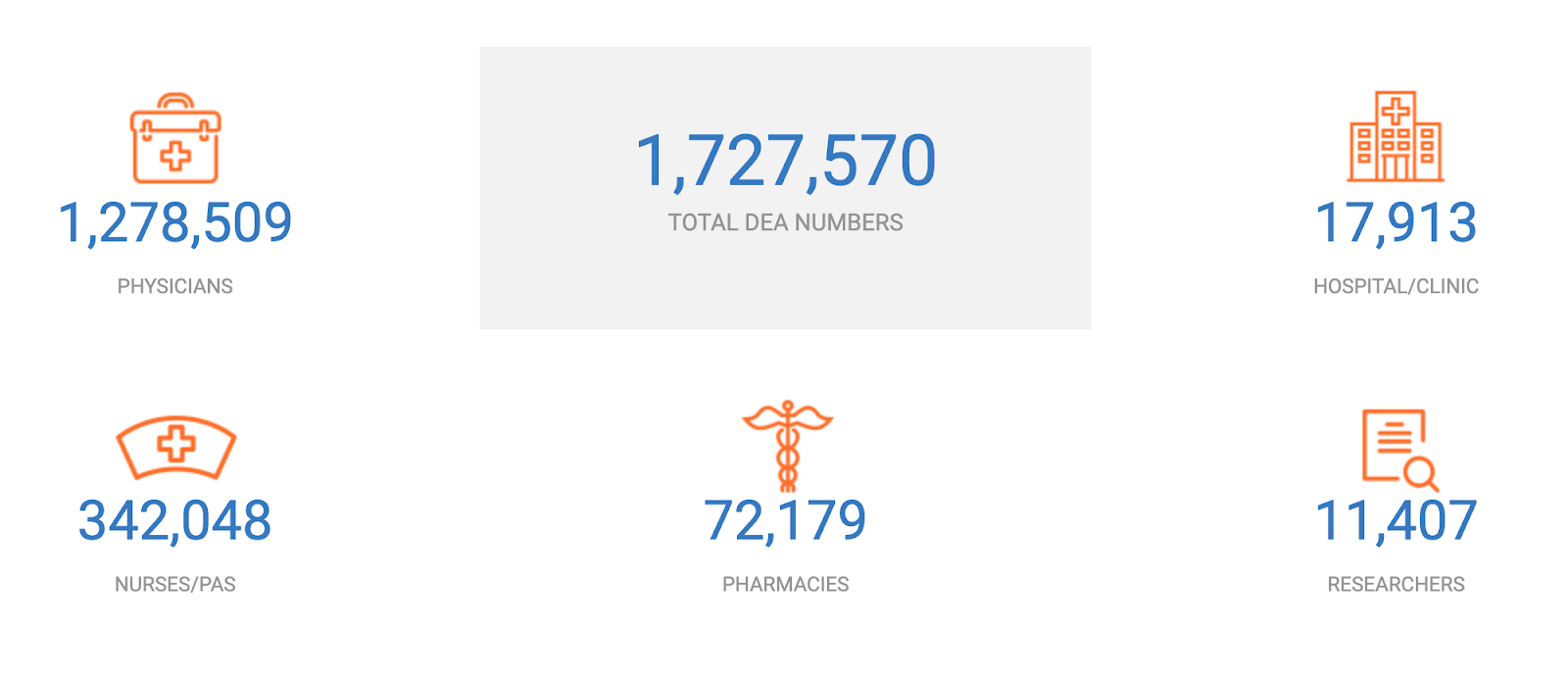
Best Practices For Dea And Fda Physician Screening Providertrust

What Is The Difference Between The Fda And Dea Quora

The Abcs Of Pharmacy Compliance Fda Dea And Epa Modern Healthcare
How Fda Distinguishes Between Clearance Vs Approval Vs Granted

What Is The Difference Between The Fda And Dea Quora

Why Do We Blame Big Pharma And Not The Dea Or Fda Opioid Settlement Tracker
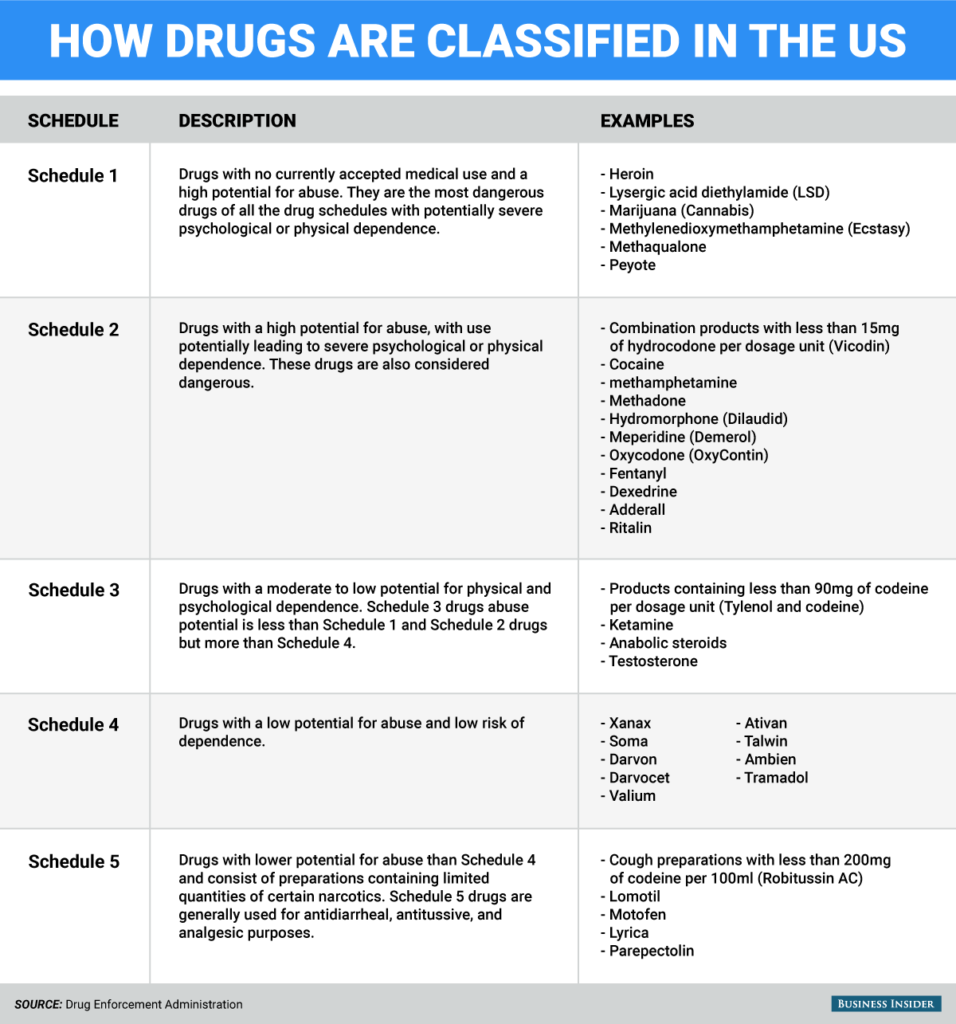
U S Controlled Drug Classifications

Best Practices For Dea And Fda Physician Screening Providertrust
Comments
Post a Comment